Iraida Franceschi

As a child, I had allergic reactions to everything and anything that grows under the sun, (i.e., grass and tree pollen, dust, mold, etc.) So I commonly experienced recurrent ear infections. Therefore, I was always given antibiotics or antihistamines, and ironically, it was also said that the medications I was taking affected my hearing loss as well. Either way, I was one of those people that thought hearing-aids were always going to be part of my life. Never imagined that hearing-aids were no longer going to be sufficient for me. Yet, during a hearing evaluation, my audiologist told me I should consider cochlear implants, and I went home feeling overwhelmed. Fast forward to 2011, and after being told I qualified, I started to research more and more about cochlear implants all the while asking questions in social media. It was all a little daunting but having most of my worrisome questions answered by my doctor and by other cochlear recipients slowly gave me confidence to go forward with the process.

In 2012, I decided to get a cochlear implant – and it was a success! Having had such a great experience with my first, I decided to go for my second a year later, becoming bilateral in 2013. At that time, I received the Nucleus 5 processors (for both sides), and I continued enjoying the journey for approximately 8-9 years until it was time to think about an upgrade. Cochlear Americas had just released their newest processor: Kanso 2. So, I knew that all I needed to do to get the upgrade process started was to email or call Cochlear, as they now have a Reimbursement and Insurance Services department to help with the process. Cochlear Americas also takes care of contacting my Audiologist and ENT Surgeon to obtain the letter of medical necessity and other information pertaining to the upgrade. Consequently, I am happy to announce that my upgrade was successfully approved, and that I have had the Kanso 2’s since November 2020.

Key Features of the Kanso 2
Built-in Rechargeable Battery: The rechargeable battery is non-removable, and internally settled (built-in) inside the Kanso 2. This means I do not need to worry about putting on or taking off the rechargeable battery, because the Kanso 2 is a one-piece unit processor. This is also known as an Off-The-Ear (OTE) processor.
Easy on and off functionality: I can turn it on by placing it on my implant and turn it off by taking it off. Or I can tap twice to turn on and tap three times to turn it off while it’s still on my implant.
SmartSound iQ with Scan Technology: The scan technology is automatically listening to the surrounding sounds, and it adapts or changes the settings depending on the environment. There are six scenes in total: Quiet, Speech, Speech in Noise, Noise, Music, and Wind.
Direct connectivity to a smartphone: Connecting to a smartphone (Android or iPhone) to stream and enjoy music, movies, videos, phone calls and video calls.
Nucleus Smart App: The app can help control the sound settings, track hearing data, and battery level. It is free and can be downloaded from the Google Play Store or Apple Store.
Dual microphones: This is important to help filter out background noise and enhance the hearing experience.
Once I received the processors, I called my audiologist to make a mapping appointment. This was necessary because the Kanso 2’s I had received are the latest and newest processors available – and were completely different from the processors that I was using.
In comparison, the Nucleus 5 is a behind the ear (BTE) processor, whereas the Kanso 2 is an off the ear (OTE) processor. This allowed me to free my ear for wearing glasses or sunglasses — and masks which are required now due to the pandemic.
With an Off-the-Ear processor, I won’t need to worry about the coil or the cable, as the Kanso 2 is a one-piece unit. Moreover, the Kanso 2 has a built-in rechargeable battery in it that is non-removable. This means that when I am done for the day, I can simply place my processors in the charger/dryer box and close the lid to allow it to automatically begin charging wirelessly. It takes approximately 3 to 4 hours to charge in full. However, because most of us sleep 6-8 hours at night, it has been said we can leave them safely drying overnight, the technology is smart, and the charger/dryer won’t overcharge the processors.
Once I go about my daily routine, and if I am attending online meetings that day, I know I can use the Mini-Mic 2+ to stream directly into the Kanso 2’s. All I have to do is plug one end of an audio cable into the headset port on my computer, and the other into the Mini Mic 2+. Then I select the audio input on the Mini Mic 2+ by pressing its mode-selector button three times to set it to the music icon. The Mini Mic 2+ streams sound wirelessly to both Kanso 2 processors, and its mic picks up my voice. This, to me, is a game changer! The sound quality is impressive, and I can follow conversations with my colleagues via Zoom. Just to be able to listen and chime in with constructive feedback, is all worth it.

I am also excited to share that working out with my Kanso 2’s has been easier. Plus, I found out that the Kanso 2’s have a water resistance rating of IP68, which means that the sound processor can withstand sweat much more than my Nucleus 5’s did as these had an IP57 rating. I could also get rained on or splashed and not have to worry about damaging my Kanso 2’s. In fact, I have been reading about the IP68 rating, and it has been said that the Kanso 2’s can be submerged underwater (to a maximum depth of 1.5m) for up to thirty minutes without any damage.
After I am done teleworking, I am usually using my iPhone, listening to music, or browsing TED Talks videos to listen to as that is now part of my daily audio rehab. With the iPhone, I don’t need any other intermediary devices, as the Kanso 2’s are able to stream sound directly to my implants. As a matter of fact, this is probably the easiest thing to do, to make calls or receive FaceTime calls from my daughters, because once the iPhone is paired, I really do not need to press any buttons. It just rings and streams without delay.
I can scroll down the timeline in social media and if I click on a video, it will instantly stream the sound to my Kanso 2’s. If I get interrupted with a call or a FaceTime call from my daughter, the call will take over and instantly connect. Then when I hang up, the video that I was watching resumes playing. It surely is as easy as I am making it sound!

The Kanso 2’s can also be used with the Nucleus Smart App, which is a free app that can be downloaded from the Apple Store – or Google Play (for Android). Once downloaded and paired, I can check my settings, such as volume, sensitivity, treble and bass – or I can change programs or leave it on Scan, which is a special program that listens to the surrounding sounds near me, making adjustments depending on whether there is noise or quiet, speech, music or even wind.
There is also a Hearing Tracker feature, which displays Time in Speech (per day) in which I can tell it to “set a goal” depending on the number of hours I wish to achieve and it will highlight the days I have met the goal. The other feature in the Hearing Tracker is the Coil-Offs for tracking the number of times the coil was disconnected from the implant each day.
If I ever misplace my Kanso 2’s, I can tap the “Find my Processor” button to show me the location of my Kanso 2’s to help me find them. So, there are quite a lot of features in the app that are useful. But the feature I use the most is the “status” of the batteries, as the Nucleus Smart App can display the amount of charging power remaining in the rechargeables, and it does so by displaying the percentage. This is a very convenient feature.
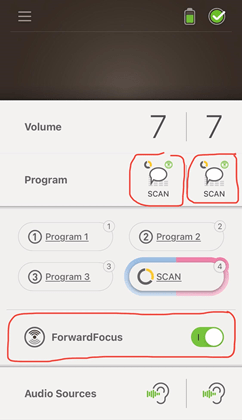
I also tested the “Forward Focus” feature, which is also a very effective setting for when I wish to drown out any noise behind me. So, I placed my back behind the TV, which was playing really loudly, and I had told my spouse to read a paragraph or two out of a book I had grabbed. He read out loud and I started to notice that his voice was in focus while the TV noise was reduced to some degree – but noticeable enough for me to enjoy this feature.
Now, in regards to the Kanso 2’s SmartSound iQ with Scan Technology, I was surprised that I didn’t notice much difference. I have tested this program by playing loud music from my Amazon Echo to make the Scan recognize music and change settings, and it certainly did so. Then I would walk away to the dining room and start a conversation with my husband, and the Scan program would automatically change to the Speech setting. All these changes were happening as I expected them to, but in a very subtle and unnoticeable way. In fact, the only way I could tell it was changing was because I was looking at the Nucleus Smart app and watching the changes. Perhaps I should give more time to the Scan feature or bring it to the attention of my Audiologist for enhancement.
Now let me bring up a different aspect about the built-in rechargeables. As mentioned, the rechargeable battery is part of the Kanso 2 — which brought up concerns (to me) about whether or not it would last me all day long. This is because I didn’t want to have to take off my Kanso 2’s in the middle of the day, or during a meeting, or even late at night while watching a movie. The difference is that the Kanso 2 would need to be removed to be charged, and I would be left without sound. However, after doing all the testing, I have come to realize that I am able to wear my processors for 2 days without charging them every single night. That’s how much power there is in the Kanso 2’s (with my Scan and Mapping programs). In the case of a power outage or when going camping, I can use the Portable Charger that was included in the upgrade kit. The portable charger will charge similar to the charger/dryer unit for approximately 3-4 hours, which would give me another 2 days to wear my Kanso 2’s. If, however, I were to go camping for a whole week, or if the power outage were to last much longer, my back up plan is to use my AC outlet portable power station. This can be purchased online and is available at outdoors sporting stores.
Either way, I can tell that the new Kanso 2’s are going to be my new connection to life. I plan to explore the new features and enjoy the new experiences my Kanso 2’s bring me. After all, this is the technology I have always dreamed of having, and I couldn’t feel any more grateful to be able to do so.
About the Author

Iraida Franceschi is a boricua wife, mother of two adult daughters, and one of the five Admins of Cochlear Town USA, a closed-group on Facebook. She mentors and corresponds with other candidates during her spare time.

 The United States Federal Communications Commission has approved the radio-frequency components of a new family of processors from Advanced Bionics. According to the report, there will be three versions, presumably offered in different markets around the world, and with different feature sets. The three processors are identified as Naída CI M30, Naída CI M60, and Sky CI M90. The accented character isn’t in the FCC documentation, but the assumption is that it will mirror the current family of processors.
The United States Federal Communications Commission has approved the radio-frequency components of a new family of processors from Advanced Bionics. According to the report, there will be three versions, presumably offered in different markets around the world, and with different feature sets. The three processors are identified as Naída CI M30, Naída CI M60, and Sky CI M90. The accented character isn’t in the FCC documentation, but the assumption is that it will mirror the current family of processors.
 The Nucleus 7 processor has received FDA approval for N22 recipients. Cochlear continues to support its legacy users by making the latest technology available to the greatest extent possible. The new RONDO 2 processor also received FDA approval, but it is not compatible with N22 implants.
The Nucleus 7 processor has received FDA approval for N22 recipients. Cochlear continues to support its legacy users by making the latest technology available to the greatest extent possible. The new RONDO 2 processor also received FDA approval, but it is not compatible with N22 implants. The new RONDO 3 has its own
The new RONDO 3 has its own 

