MED-EL’s Sound Sensation Music Festival Celebrates International Artists with Hearing Loss
October 6, 2022 – (Durham, NC) – MED-EL, a global leader in implantable hearing solutions, has been passionate about connecting people with hearing loss to the magic of music since pioneering the cochlear implant in 1977. This year, between October 6-8, MED-EL will bring music alive at their Sound Sensation Festival. Singers and musicians from around the world who are deaf and hard of hearing and rely on MED-EL technology to hear will perform alongside renowned artists including a Vienna Philharmonic ensemble, violinist Yury Revich, and Eurovision winner Conchita Wurst, demonstrating the power of music in bringing people together.
To many, enjoying the multifaceted aspects of music is an integral part of life. Giving musicians with hearing loss a stage aims to inspire cochlear implant users around the globe. The virtual festival is for everyone who defies expectations with their hard work and natural talent to make world-class music.
“When people lose their hearing, they tell us that they miss the sound of family and friends, birds and music. Many of our incredible users go on to enjoy and play music again. Our festival is a celebration of their love for music and life. We see so many MED-EL users accomplish greatness in a multidimensional world of sound and music and strongly believe the world should see so too,” said Marcus Schmidt, Director of Corporate Marketing at MED-EL.
Organized and hosted by MED-EL, the virtual event will be the world’s biggest music festival the hearing implant community has seen. It will bring together individuals with hearing loss, their families, and ENT professionals as part of a series of regional and global online events. Small-scale concerts championed by hearing implant users, workshops, a symposium for hearing professionals will occur, followed by a live Grand Finale that will take place on October 8.
The three-day festival will feature a comprehensive program for everyone with a love for music. Viewers interested in listening to music and musical rehab can experience:
• Interactive workshops for parents, implant users and rehabilitation specialists
• Short daily concerts featuring musical performances from countries across the world
• A “Best Of” event showcasing a wide variety of MED-EL users’ musical activities
The entire festival will be broadcast globally and will be available on demand. For more information, please visit the event page here.

 The United States Federal Communications Commission has approved the radio-frequency components of a new family of processors from Advanced Bionics. According to the report, there will be three versions, presumably offered in different markets around the world, and with different feature sets. The three processors are identified as Naída CI M30, Naída CI M60, and Sky CI M90. The accented character isn’t in the FCC documentation, but the assumption is that it will mirror the current family of processors.
The United States Federal Communications Commission has approved the radio-frequency components of a new family of processors from Advanced Bionics. According to the report, there will be three versions, presumably offered in different markets around the world, and with different feature sets. The three processors are identified as Naída CI M30, Naída CI M60, and Sky CI M90. The accented character isn’t in the FCC documentation, but the assumption is that it will mirror the current family of processors.
 The Nucleus 7 processor has received FDA approval for N22 recipients. Cochlear continues to support its legacy users by making the latest technology available to the greatest extent possible. The new RONDO 2 processor also received FDA approval, but it is not compatible with N22 implants.
The Nucleus 7 processor has received FDA approval for N22 recipients. Cochlear continues to support its legacy users by making the latest technology available to the greatest extent possible. The new RONDO 2 processor also received FDA approval, but it is not compatible with N22 implants. The new RONDO 3 has its own
The new RONDO 3 has its own 

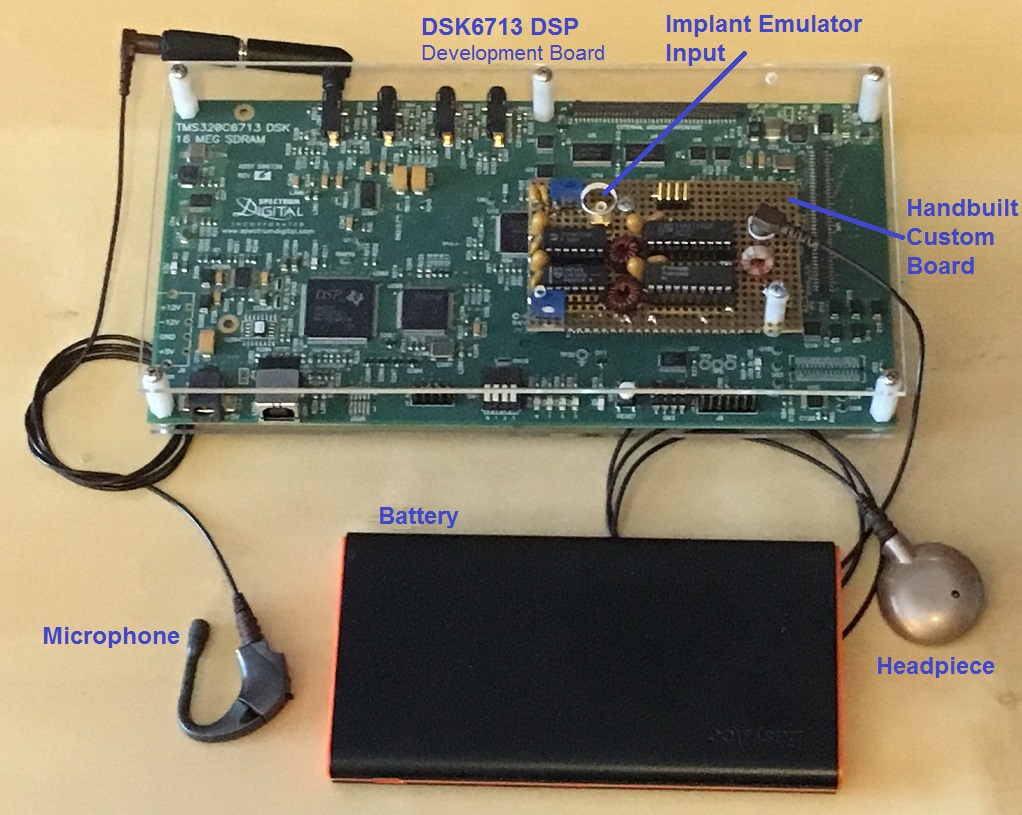
 Suzy & Mike live an hour north of San Francisco. Mike is a retired telecommunications engineer from Hewlett Packard. He is an adjunct professor at Arizona State University in the Speech and Hearing Sciences, and works with both the ASU and UCSF CI teams trying to improve low frequencies and music for CIs using his hand-built speech processor.
Suzy & Mike live an hour north of San Francisco. Mike is a retired telecommunications engineer from Hewlett Packard. He is an adjunct professor at Arizona State University in the Speech and Hearing Sciences, and works with both the ASU and UCSF CI teams trying to improve low frequencies and music for CIs using his hand-built speech processor.