Inaugural Hearing Health & Technology Matters Awards Recognize SYNCHRONY Cochlear Implant System, BONEBRIDGE Bone Conduction Implant and AudioKey App for Innovation Excellence
DURHAM, NC – October 19, 2020 – MED-EL announced today that it has been named Innovator of the Year by the first-ever Hearing Health & Technology Matters (HHTM) Innovator Awards™. The announcement was made at the virtual Academy of Doctors of Audiology meeting, AuDacity 2020
MED-EL placed in each category that it entered:
- The SYNCHRONY Cochlear Implant (CI) System, was awarded 1st place (Gold) in the Auditory Implant Category. The industry-leading innovation of a diametrically-opposed magnet shattered traditional CI design features and – for the first time – enabled safe MRI with the internal magnet left in place by following simple scanning instructions that are easy for any MRI facility. The SYNCHRONY CI System was the first, and remains the only, cochlear implant system in the United States to receive U.S. Food and Drug Administration (FDA) approval for a single-sided deafness (SSD) indication, filling a major unmet need in the hearing loss community. The SYNCHRONY CI System now features the latest FDA approved off-the-ear audio processor, the RONDO 3.
- The BONEBRIDGE Bone Conduction Implant, was awarded 3rd place (Bronze) in the same Auditory Implant Category. The introduction of BONEBRIDGE meant that for the first time, bone conduction implant candidates could choose a system that offered a powerful combination of intact skin, enhanced wearing comfort and direct stimulation of the bone. As a result, BONEBRIDGE is widely considered a breakthrough in bone conduction technology and it aligns with the MED-EL mission to gently restore hearing with intact-skin hearing implants that enhance communication and quality of life.
- The AudioKey App, was awarded 2nd place (Silver) in the Assistive Technology & Software Category. AudioKey is the only cochlear implant app that allows a parent to pair multiple user’s audio processors with their phone, which is ideal in families with more than one MED-EL cochlear implant recipient.
“The Innovator of the Year award is an exciting accolade, as innovation is a part of our DNA. While we are driven by research and development, MED-EL is so much more than a single implant, processor or technology. We are hundreds of thousands of lives that have been changed as a result of the pursuit of ideas that were once thought to be impossible,” said Raymond Gamble, President & CEO, MED-EL North America. “It’s gratifying to be recognized for our pioneering vision and dedication to fulfilling our mission.”
“It is exciting to recognize and honor companies who invest in innovation to improve the lives of people with hearing loss. We are thrilled to name MED-EL as our first-ever Innovator of the Year based on their industry leadership and innovative spirit,” said Kevin Liebe, AuD, HHTM President and CEO.
Through the annual awards program, HHTM recognizes technological innovation and achievement in the hearing industry. This year’s awards program saw dozens of innovative technologies, submitted from companies across the globe.
About the Hearing Technology Innovator Awards
The Hearing Technology Innovator Awards™ is an international awards program designed to recognize and celebrate innovation within the hearing industry. Learn more here.
About Hearing Health & Technology Matters
Hearing Health & Technology Matters (HHTM) is an organization dedicated to bridging the knowledge gaps in treating hearing loss. Readers and contributors are drawn from many sectors of the hearing field, including practitioners, researchers, manufacturers, educators, and, importantly, consumers with hearing loss and those who love them. By involving all these groups, we hope to bridge the gaps in knowledge that so often divide them. To learn more, visit HearingHealthMatters.org.
About MED-EL
MED-EL Medical Electronics, a leader in implantable hearing solutions, is driven by a mission to overcome hearing loss as a barrier to communication. The Austrian-based, privately owned business was co-founded by industry pioneers Ingeborg and Erwin Hochmair, whose ground-breaking research led to the development of the world’s first micro-electronic multi-channel cochlear implant (CI), which was successfully implanted in 1977 and was the basis for what is known as the modern CI today. This laid the foundation for the successful growth of the company in 1990, when they hired their first employees. To date, MED-EL has grown to more than 2,200 employees from around 75 nations and has 30 locations worldwide.
The company offers the widest range of implantable and non-implantable solutions to treat all types of hearing loss, enabling people in 124 countries to enjoy the gift of hearing with the help of a MED-EL device. MED-EL’s global portfolio of hearing solutions includes cochlear implant systems, a combined Electric Acoustic Stimulation hearing implant system, and bone conduction devices. www.medel.com



 The United States Federal Communications Commission has approved the radio-frequency components of a new family of processors from Advanced Bionics. According to the report, there will be three versions, presumably offered in different markets around the world, and with different feature sets. The three processors are identified as Naída CI M30, Naída CI M60, and Sky CI M90. The accented character isn’t in the FCC documentation, but the assumption is that it will mirror the current family of processors.
The United States Federal Communications Commission has approved the radio-frequency components of a new family of processors from Advanced Bionics. According to the report, there will be three versions, presumably offered in different markets around the world, and with different feature sets. The three processors are identified as Naída CI M30, Naída CI M60, and Sky CI M90. The accented character isn’t in the FCC documentation, but the assumption is that it will mirror the current family of processors.
 The Nucleus 7 processor has received FDA approval for N22 recipients. Cochlear continues to support its legacy users by making the latest technology available to the greatest extent possible. The new RONDO 2 processor also received FDA approval, but it is not compatible with N22 implants.
The Nucleus 7 processor has received FDA approval for N22 recipients. Cochlear continues to support its legacy users by making the latest technology available to the greatest extent possible. The new RONDO 2 processor also received FDA approval, but it is not compatible with N22 implants.

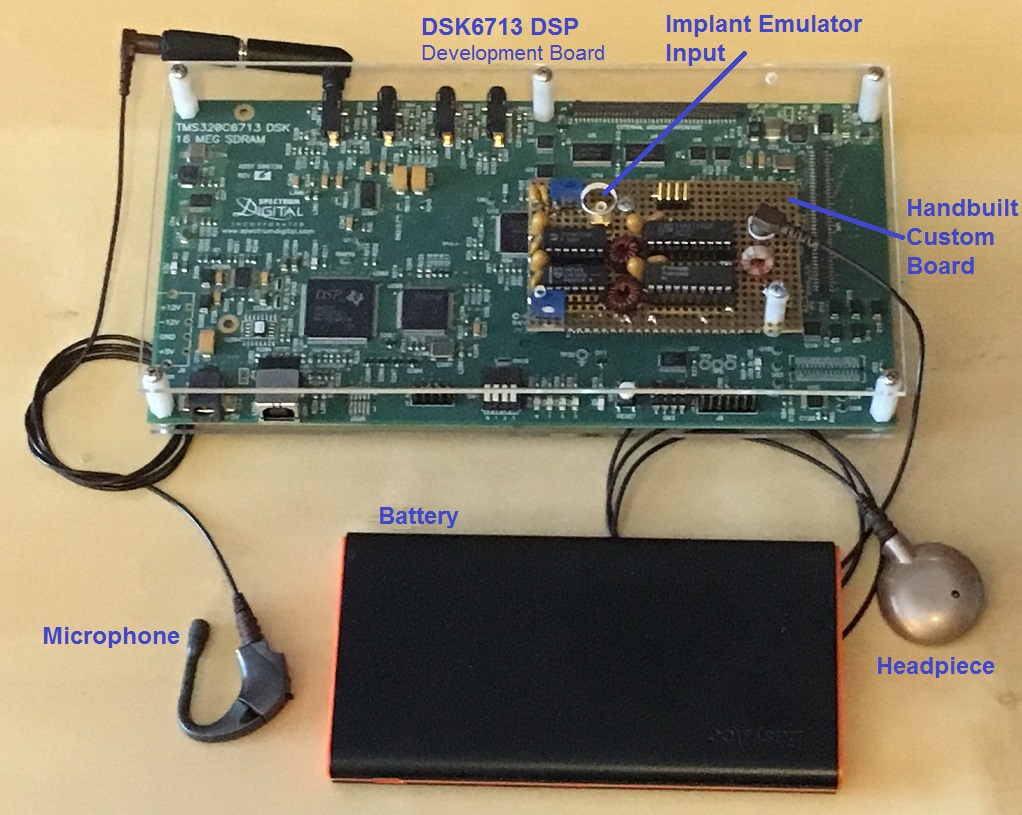
 Suzy & Mike live an hour north of San Francisco. Mike is a retired telecommunications engineer from Hewlett Packard. He is an adjunct professor at Arizona State University in the Speech and Hearing Sciences, and works with both the ASU and UCSF CI teams trying to improve low frequencies and music for CIs using his hand-built speech processor.
Suzy & Mike live an hour north of San Francisco. Mike is a retired telecommunications engineer from Hewlett Packard. He is an adjunct professor at Arizona State University in the Speech and Hearing Sciences, and works with both the ASU and UCSF CI teams trying to improve low frequencies and music for CIs using his hand-built speech processor.